A recent article in a terrific reoccurring physician’s column in the Duluth Reader titled, “14 Lies that Big Pharma and Their Academic Psychiatrists Teach Medical Students” that details the many fables and fairy tales Americans believe about what the FDA does and doesn’t do. (Hint: What it doesn’t do is insure drugs and devices are safe for use.) This particular article is about the delusions Americans regarding the safety and effectiveness of the drugs and devices approved by the FDA. Americans are weird in that they distrust their government for the easy stuff and are as gullible as newborn babies about the hard stuff. Dr. Gary Kohls article in the Duluth Reader bursts some bubbles about the FDA’s part in the mental health drug business and that is a pretty important story. He describes the lack of sophistication in the actual drug development, testing, and application while pointing out how much better Big Pharma’s marketing and politics are than their science. "The so-called Selective Serotonin Reuptake Inhibitors have been deceptively mis-named by Big Pharma because those amphetamine molecule-based SSRI drugs do NOT mess only with serotonin neurotransmitter systems! In fact, they do not actually raise total brain serotonin levels as advertised. Actually, SSRI drugs deplete serotonin long-term while only “goosing” the release of serotonin at the synapse level while at the same time interfering with the storage, reuse and re-cycling functions of the serotonin synapses that, by the way, are far more abundant in the human intestinal tract than in the central nervous system." You wouldn’t get that from any of their ads, would you?
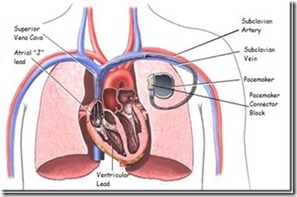
Telectronics’ product failures were eventually exposed when an autopsy of a young woman who died from the atrial carving revealed a piece of wire sticking out of the atrial “J” wire’s insulation. The pathologist, a friend of the cardiologist who performed the pacemaker implant, described the wire to the cardiologist; assuming his friend had accidentally left a guiding stylet in the atrial wire. The cardiologist was absolutely certain that wasn’t the case and asked for further investigation, risking his own reputation in the process. The resulting investigation eventually involved the FDA investigators and the discovery that several similar incidents had occurred in the past, with no certain knowledge of how many such failures had resulted in patient mortality and morbidity. That knowledge and the discovery that the company had covered up the knowledge of both types of failure brought the full attention of the FDA. At that time, Dr. David Kessler was the commissioner of the FDA and he took the job very seriously. Appointed by Bush I and replaced in 1997 by Clinton, with practically nothing of substance from then on, Dr. Kessler ran an FDA that was both active and patient-oriented. After Kessler’s FDA, American patients have been pretty much on their own as far as anything resembling federal regulation of food and drugs.
One of the experiences I had with Telectronics was in a team of folks who were writing the clinical trial plan for the company’s ICD products. I got to study chemotherapy and cardiac therapy drug trials and their methods and results. The results were every bit as interesting as the methods, since the results were so ambiguous that I don’t know how anyone could conclude either of those drugs worked worth a damn. Statistically, every clinical trial we looked at demonstrated that the state-of-the-art drugs were inconsistently the equivalent of placebos. Sometimes, the drugs were slightly worse. As Dr. Kohls explained with his, “Myth # 3: ‘FDA approval means that a psychotropic drug is safe long-term’” In fact, FDA approval means nothing. Drugs and devices "are usually only tested in human clinical trials for a couple of months before being granted marketing approval by the Big Pharma-conflicted FDA. " FDA approval is just a stamp applied to practically anything the medical industry wants to foist off on patients and medical providers.
The next train wreck, I mean medical devices company, was CPI/Guidant. When I first applied, it was still CPI, the company that invented the ICD. CPI had a reputation for being “conservative,” in the real definition of that word. Their devices (pacemakers and ICDs) weren’t tricky, but they were reliable, had long battery lives, and had only the features necessary to do the job well. CPI was acquired by Eli Lilly corporation in 1978 and Lilly added a pile of medical devices company to its portfolio until 1994 when it spun those companies off into Guidant/CRM and the rest of the mess that became Guidant. I interviewed in 1995 and early 1996 and by the time I started work in March of 1996, Lilly had separated itself from Guidant and the retirement and pension package I’d been promised vanished without anyone bothering to tell me about it. Since Telectronics was being pulled to pieces for the patents and valuable employees, it probably wouldn’t have made a difference, but it would have been nice to know what I was getting into.
What I was getting into was a carbon copy of the situation I’d just left. Guidant eventually collapsed under its own avarice and incompetence. To make a long, painful story short, one “Quality Committee” meeting illustrated the company’s philosophy perfectly. When a team of quality assurance engineers presented their analysis of a defective device that needed to be notified on, the executives in the committee asked, “How will that affect our bonuses this quarter?” Since the device failure was a life-threatening malfunction, no one had done the necessary research to determine how that would affect executive compensation. Imagine that.
Eventually, and after hundreds of patient injuries and a few deaths, a few doctors began to suspect their corporate beneficiary was less than benevolent. Even weirder, a couple of those doctors did the minimal research necessary to discover that even their own small group of patients had been affected multiple times by Guidant product failures. I had collected more than 600 such failures for one of the products I monitored and I waited for more than a year for one patient-oriented cardiologist to ask me, “Have you ever seen this before.” Never happened.
When Guidant died, 95% of the company’s products were under FDA Class 1 recall, but the FDA deserves little-to-no credit for uncovering the company’s many problems. Like the physicians profiting from selling these defective products, the FDA’s attitude was summed up by something an “investigator” said to me not long before I quit medical devices, “We don’t care what kind of product problems you have or how many patients you kill as long as we don’t read about it in the paper first.” As long as the FDA wasn’t embarrassed by being shown up by a media investigation, they will let Pharma and Devices do whatever the hell they want with patient safety and therapy effectiveness. To them, patients are nothing more than “lab rats who clean their own cages.”
No comments:
Post a Comment